Phase 1 ACTIVATE trial - 4-fold increase in tumour shrinkage
ACTIVATE was a multi-center, single arm, open-label Phase 1 trial to assess the safety and tolerability, pharmacokinetics, and preliminary anti-cancer activity of ACT® treatment with the chemotherapy regimens FOLFOX or FOLFIRI in patients with liver metastases of colorectal origin (NCT04021277).
ACTIVATE was led by Professor Udai Banerji from the Institute of Cancer Research, The Royal Marsden Hospital, UK as coordinating investigator, and Professor Ruth Plummer at Freeman Hospital, Newcastle, UK.
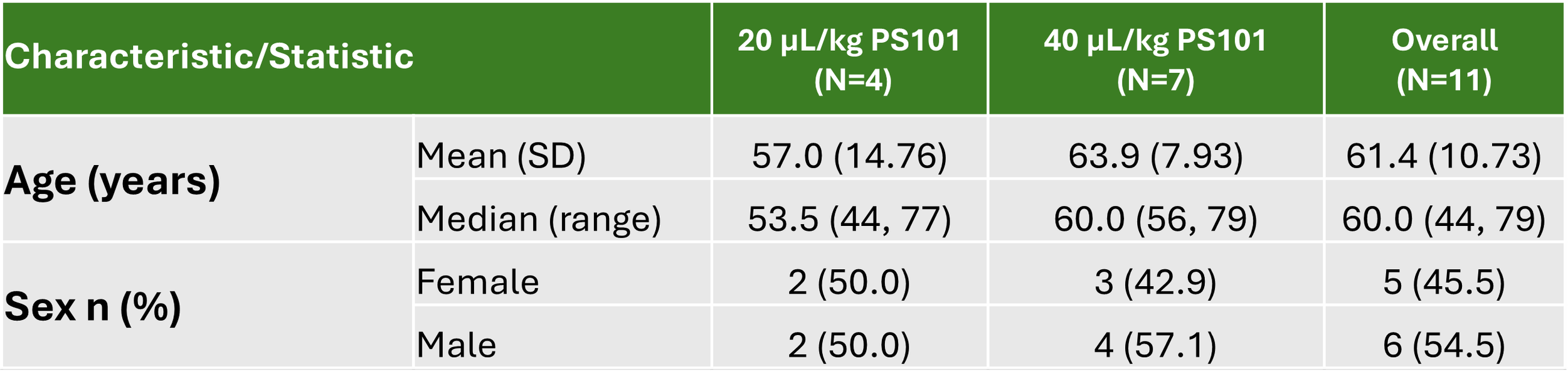
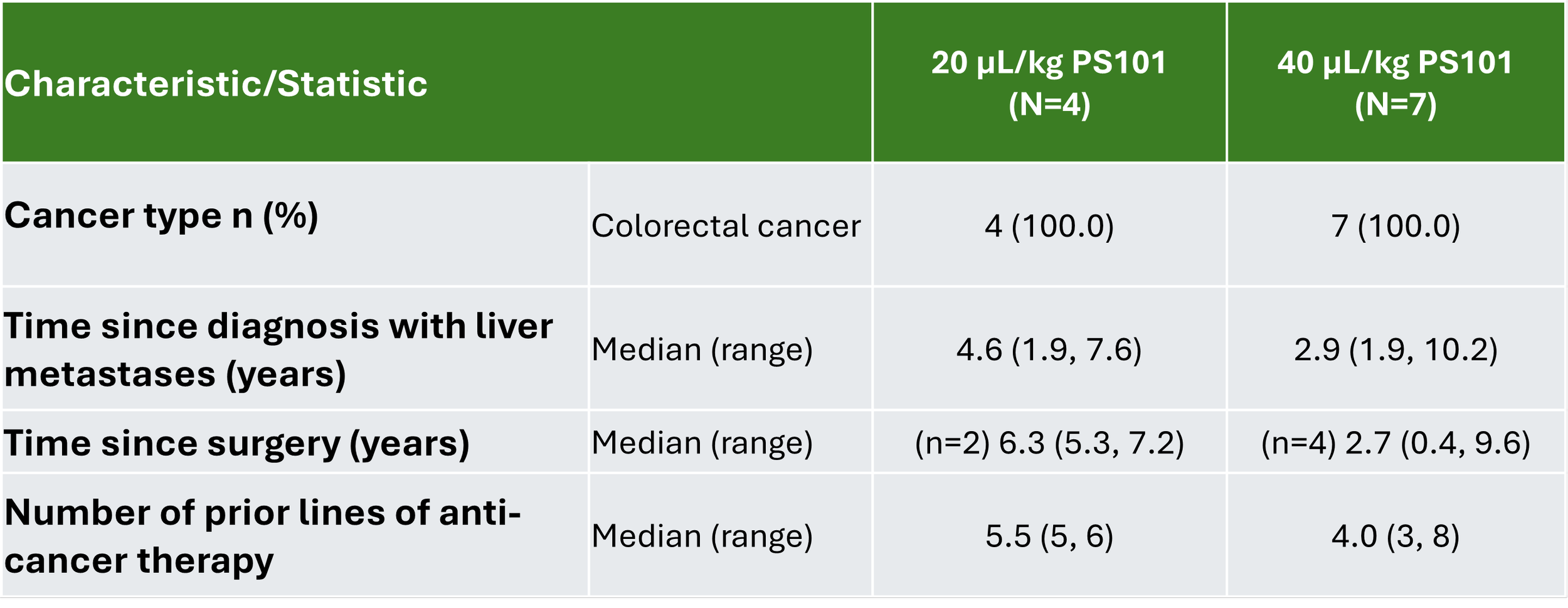
The trial enrolled 11 hard-to-treat patients, hereof 9 evaluable, at sites in the United Kingdom. Please see tables below.
Chief Medical Officer of EXACT Therapeutics, Dr. Amir Snapir:
"The final ACTIVATE read-out marks the successful completion of the ACTIVATE trial. The results give us great confidence in the treatment
potential of the ACT technology for patients with solid tumours."
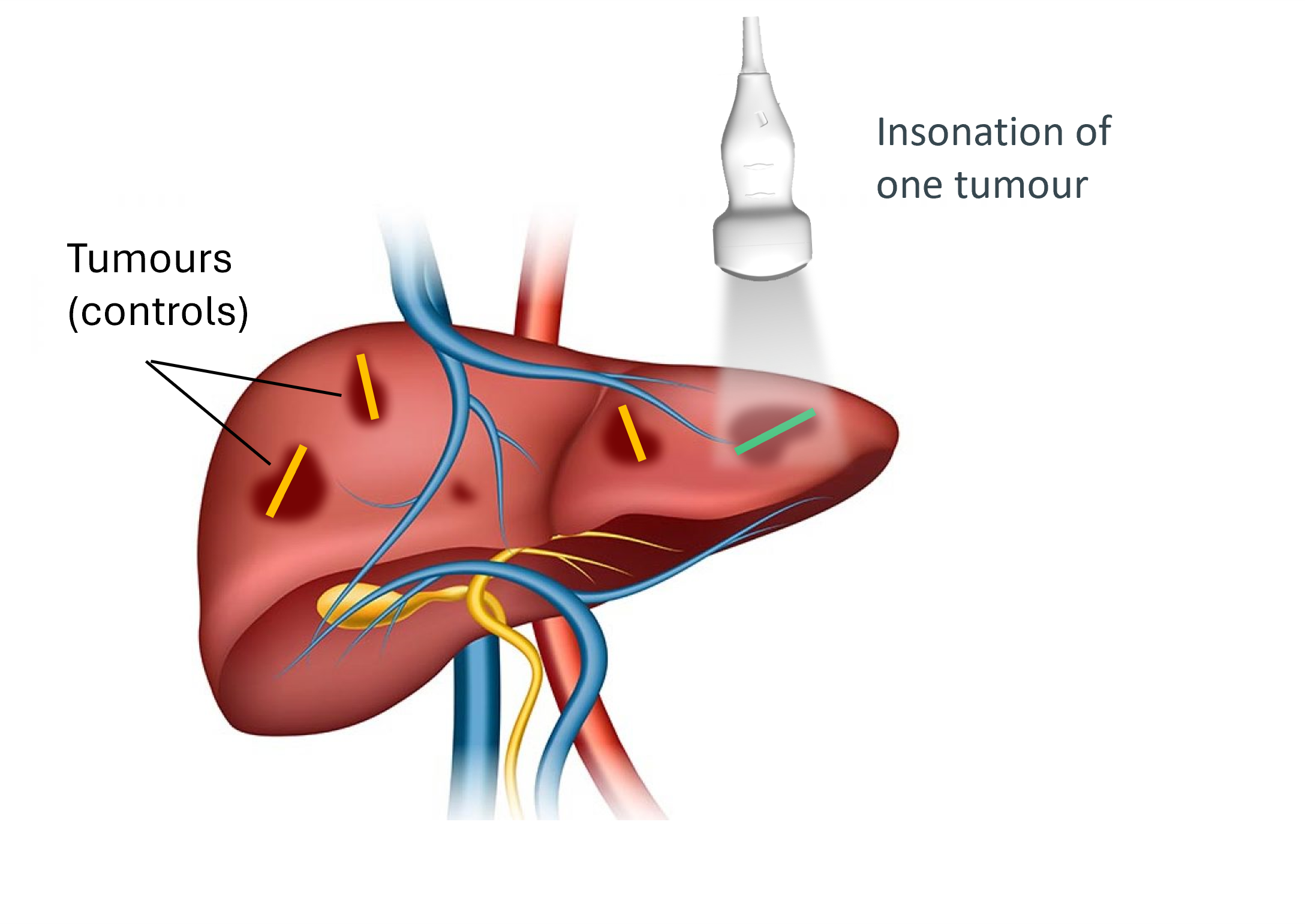
ACT treatment with EXACT’s proprietary agent PS101 had a clear dose-response relationship. Shrinkage of tumour lesions was significantly greater in patients who received 40 µl/kg PS101 compared with 20 µl/kg PS101. In the group of patients who showed a response to chemotherapy in the control lesions, 3 out of 4 patients who received 40 µl/kg PS101 showed tumour shrinkage of more than 30% in diameter, as assessed by central review. A decrease of 30% in the sum of overall tumour diameters is considered a partial response in RECIST 1.1. Please see figure above.
In the patients who had a response to chemotherapy, tumours treated with ACT showed a statistically significant greater shrinkage and 4-fold shrinkage compared to chemotherapy alone in the same individuals (−29% vs. −7%, p<0.05). Tumour shrinkage was seen in 6 out of 9 evaluable patients who received ACT with chemotherapy. PS101 was safe and well tolerated when given with chemotherapy.
Change in tumour diameter from baseline to week 8 of treatment for each patient, showing both control and ACT treated tumors
In the study, we used an innovative approach to assess within-patient effect of the combination of ACT with chemotherapy against chemotherapy alone by insonating one cancer lesion in the liver and comparing change in tumour size and volume with tumour lesions that have not been insonated. Assessment was done at week 8 by blinded central review.
Summary of Key Demographics and Baseline Characteristics
EXACT presented the final results from the ACTIVATE study at the European Society for Medical Oncology (ESMO) Congress 2025. The poster can be found here: https://exact-tx.com/publications-1-1
Poster #: 4176
Title: First-in-Human Study of Acoustic Cluster Therapy consisting of PS101 combined with Chemotherapy and insonation in Patients with Liver Metastases of Colorectal Cancer Origin
The ENACT study
EXACT has initiated a Phase 2 trial (NCT06850623) where the safety and efficacy of PS101 will be investigated in combination with standard of care as 1st line treatment in patients with borderline resectable or unresectable locally advanced pancreatic cancer – the ENACT trial.
January 2026, EXACT published the a positive initial safety read-out and early, encouraging tumour shrinkage, and significant decrease in tumour biomarker CA 19-9 (>85% decrease, unaudited data). Interim efficacy read-out is expected mid-2026. The trial monitoring committee issued their statement on safety on January 27, 2026 - link
Summary of Disease History